Fullerene is a group of carbon molecules that usually form a kind of carbon tube, these are used especially for nanotechnology. In the following article we will know everything about this and much more.
What is Fullerene and what are its uses?
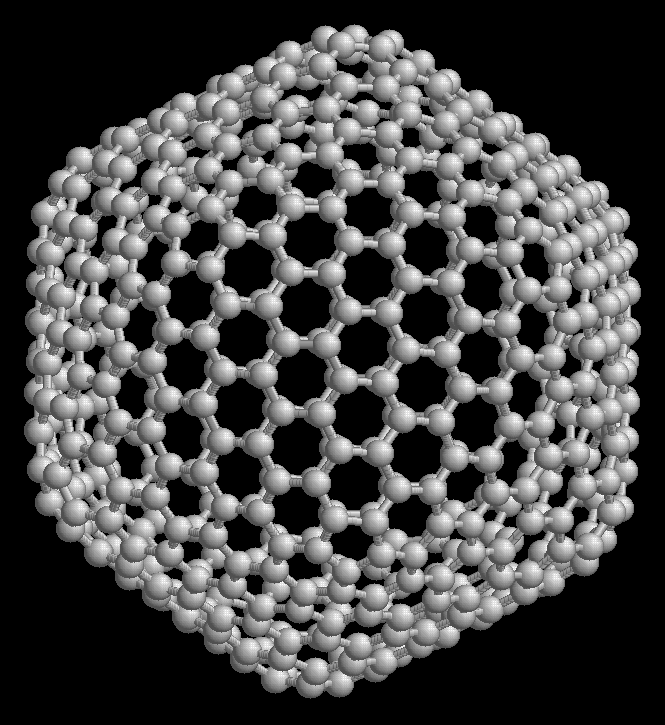
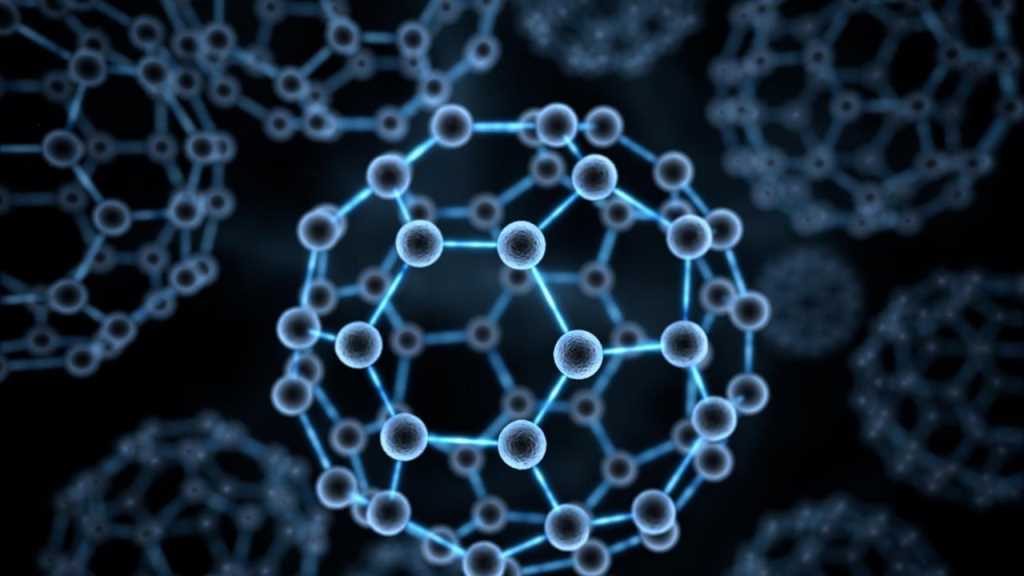
The so-called Fullerene, which is also known as "Buckminsterfullerene", consists of a series of empty carbon molecules that form a kind of closed cage called "buckyballs" or a kind of cylinder that are carbon nanotubes.
Fullerenes are usually a class of carbon molecule with a particular kind of construction that employs physical ways such as a type of sphere or tube. Said molecules in the same way can have shapes such as hexagonal and also pentagonal. However, what is fullerene and what is it for? Fullerenes are a class of elements useful in certain types of computing applications, especially in the building sciences called nanotechnologies.
Fullerene History
A Fullerene was found in 1985 by a group of people named Richard Smalley, James Heath, Robert Curl, Sean O'Brien and finally Harold Kroto while at Rice University. Said first fullerene managed to be discovered in the name of buckminsterfullerene scientifically called "C60", and its name paid homage to Buckminster Fuller. Robert Curl was the person who won the Nobel Prize for the discovery of fullerenes in 1996.
However, the discovery of the so-called "Bucky-ball" has been led by research on a kind of new class of materials that have been cataloged as fullerenes, or as the "buckminsterfullerene" which is the one that refers to the smallest fullerene . As we already know from certain allotropes of carbon, which are limited to mineral elements such as:
- Diamonds
- Graphite
- Nanotubes
- Coal
- Amorphous Carbon
The discovery of the so-called "bucky-balls" was what significantly elongated carbon allotropes and has become the subject of a kind of passionate research within the field of microelectromechanical systems known by its acronym "MEMS", consisting of:
- Materials Sciences
- The electronic
- nanotechnology
The various studies are the ones that have revealed that the type of work of fullerene is one that is largely based on the various theoretical and experimental systems.
Fullerene Structure
Fullerenes are similar in their structure to graphite, which is composed of a kind of sheet of hexagonally linked rings, however, they contain pentagonal rings or on many occasions as heptagonal that prevent the sheets from being flat.
Fullerenes have sp2 and sp3 hybrid carbon atoms. These molecules have a very high affinity class for electrons and are the ones that can be reversibly reduced to absorb electrons.
Despite the fact that said molecule is made by the carbon rings that have been conjugated, the electrons on this occasion are not delocalized, for which these same molecules are the ones that lack the property of superomaticity. The same molecules harbor a class of very high tensile strength and are the ones that recover their original shape after being subjected to more than 3 atmospheric pressures.
This because of the unique properties of said allotrope of carbon, so they have a class of applications. Because of relativity to ease of synthesis, the so-called Fullerene C60 It continues to be very popular and a great deal of research has been carried out for its applications at a higher level.
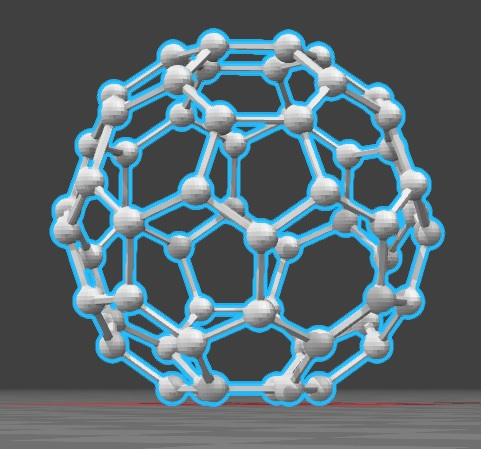
The fullerene C60 is made up of about 60 carbons in about 60 vertices that are the ones that form a kind of spherical structure. This is made up of about 12 rings that are hexagonal that are usually adjacent to each other. Said rings are being conjugated with double bonds.
The CC junction length for hexagonal rings is typically about 1,40 A° and about 1,46 A° for pentagonal rings, with a mean junction length class equal to the 1,44 A°
Types of Fullerene
Fullerenes have many kinds of structural variations, and they have made excellent progress in 1985. These that we are going to describe are some examples of the types of Fullerenes that work well:
Nanotubes or Cylindrical Fullerenes
These have a hollow shape, of dimensions that are extremely minimized. Nanotubes found to be made of carbon are generally wide and can be differentiated from a few nanometers to many mm (millimeters) in length. They have one end closed and the other open.
The electronics industry is the one that mainly uses carbon nanotubes, another area is space technology in order to be able to produce the high resistance carbon cables which are necessary for space elevators and for spacecraft cases. paper batteries.
Bunches of Buckyballs
This is the minimum fullerene found in nature. The smallest member of this is the dodecahedron and the most common consists of the C60 which is the icosahedron that is similar to a soccer ball, composed of about 20 hexagons and 12 pentagons. The small fullerene is of great importance in terms of natural occurrence, and it can be found in soot or even coal.
The Megatubes
As its name indicates, this is the Mega, which means Large, they have tubes that are much larger in diameter than in the case of nanotubes. The walls of the megatubes are being prepared with different thicknesses. Said kinds of tubes are fundamentally used in the transport of a variety of molecules of different dimensions.
polymers
These are called macromolecules that are connected by covalent chemical bonds. The so-called polymers are essentially made by carbon chains. Under high pressure and at high temperatures they usually form two-dimensional polymers and also three-dimensional ones.
The Nano – Onion
This consists of a solid Buckyball shape, with spherical shaped particles which are based on multiple layers of carbon.
The “Ball and Chain” Dimers United
These are two balls of buckyballs that are being held together by a single carbon chain.
The Fullerene Rings
The last type of Fullerene that remains to be described are the Fullerene Rings, however, there is not much information about these, only that it is formed by a ring or ring of fullerenes buckyballs.
Fullerene Uses – Applications
With the beginning of the so-called "Nanotechnology" various things have been presented to the whole world. The so-called Fullerenes are the ones that acquired the main focus in the field of nanotechnology. The great space organization called NASA, in collaboration with the renowned geochemist Lynn Becker, managed to discover the fullerenes that are created naturally.
Because of the unique chemistry in the material sciences, great researchers have been able to discover the various applications of fullerenes, which are to include medical applications, optical fibers, and superconductors.
Antioxidants
Fullerenes are excellent producers of antioxidants, this kind of property is what can be attributed to a number of conjugated double bonds that they have and also to a kind of very high electronic affinity of said molecules, this because of the energy of the molecular orbit which is low and unoccupied. Fullerenes can react with chain radicals long before they are consumed.
Antiviral Agents
Fullerenes have always attracted attention because of their strength as excellent antiviral agents. Perhaps its appearance is much more exciting, in this regard, which may be due to its ability to eliminate the replication of the Human Immunodeficiency Virus, popularly known as "HIV", and for this, it helps to delay the presence Acquired Immunodeficiency Syndrome known by its acronym "AIDS".
It has been observed that dendrofellerene 1 and its derivative 2, which is the trans isomer, are the ones that inhibit the protease class of the HIV virus and, therefore, prevent the replication of HIV 1 itself.
Drug Delivery and Gene Delivery
The administration of drugs becomes the transport of a type of pharmaceutical compound to the site of action, while the administration of genes consists of the introduction of foreign DNA inside the cells in order to be able to produce the drug. desired type of effect.
Therefore, it is of great importance to deliver these molecules with the utmost safety and efficiency. Fullerenes are a class of inorganic carriers, these classes of molecules are often favored because they have shown excellent compatibility, including higher selectivity, they retain what is biological activity, and they are as small as possible to be extended. .
Photosensitizers in Photodynamic Therapy
Photodynamic therapy known by its acronym "PDT" consists of the form of therapy that makes use of a type of compound that is sensitive to light and that is not toxic, this when it is placed in light, then if it becomes toxic. It is used to treat malignant or altered cells. Fullerenes are generally used for these classes of compounds.
In Safety Glasses
Fullerenes have limited optical properties. This refers to their ability to reduce the transmittance of the light that falls on it. Said molecules can, therefore, be used as a kind of optical limiter that is used in goggles or protective and sensor lenses.
Fullerene Properties
We are going to present what are the main properties of Fullenero on a physical level.
Physical Properties of fullerene C60
- The density: It is 1,65 g cm-3
- The Standard Heat of Formation: It is 9,08 kcal mol-1
- Refractive index: It is 2,2 (600nm)
- Boiling point: It is Sublime at 800 K
- Resistivity: About 1014 ohms m-1
- Vapor Density: N/A
- Crystal Shape: N/A
- Hexagonal Cubic Vapor Pressure: 5 x 10-6 torr at room temperature : 8 x 10-4 torrential at 800 K
- Organoleptic Properties: It has the appearance of balloon soot: very finely divided black powder
- The Fullerites: A brown/black powder
- C60: solid black
- The smell: Toilet
Fullerenes in Space
As we have already said, fullerenes are usually formed "Coiled" in a sheet of graphite and adding some particles of pentagons to achieve its curvature. If the sheet is only rolled up as a kind of cylinder, then they must cover the corners with curved hemispheres with pentagons. What will be obtained a carbon nanotube.
Another article that is recommended to study are the Contributions by Blaise Pascal which are often useful for the procedures of this element. These types of materials are usually very different from the materials of the fullerene class – type, in short, to the Round Cages and therefore have very different properties.