Uyazi ukuthi yini ukuhlolwa kwe-hertz? Kwakuwucwaningo olwenziwa okokuqala ngo-1914 ngososayensi uJames Franck noGustav Ludwig Hertz, inhloso yabo kwakuwukusungula isilinganiso samazinga wamandla ama-electron akhona kuma-athomu.
Ukuhlolwa kuka-Franck no-Hertz
Ukuhlola kuka-Hertz kwakwazi ukuqinisekisa imodeli ye-quantum ye-athomu ka-Bohr, okufakazela ukuthi ama-athomu ayekwazi ukumunca inani elithile lamandla elibizwa ngokuthi i-quanta. Ngaleso sizathu, lokhu kungenye yezivivinyo ezibalulekile ze-quantum physics. Ngenxa yalolu cwaningo, uFranck noHertz baklonyeliswa ngeNobel Prize in Physics ngo-1925.
Umlando, Wayengubani u-Hertz?
Ngonyaka ka-1913, u-Niels Bohr wakhuthaza ukuba khona kwemodeli entsha ye-athomu, kamuva eyabizwa ngokuthi i-athomu. Isibonelo se-Bohr Atomic, futhi waphakamisa ukuba khona kwama-electron orbits, okwakunemodeli ye Rutherford Atomic Model, njengesimiso samaplanethi. Ngemodeli yakhe waphakamisa ama-postulates amane, enye yawo eyayihlobene nokulinganisa kwemigqa yama-electron.
Ngale ndlela, ukuhlola kokuqala kwakuhloswe ukukwazi ukuqinisekisa lokhu kulinganisa. Ekuhloleni kokuqala, ukukhanya kwasetshenziswa, njengoba ngaleso sikhathi kwakwaziwa ukuthi ukukhanya kwakhiwe i-quanta yamandla. Ngenxa yalesi sizathu, i-Bohr igxekwa ngenxa yokuthi imiphumela ye-quantization ye-orbits, ngakho-ke, ye-quantization yezifunda zamandla zama-electron e-athomu, yayinomsuka wayo kuphela ekukhanyeni kokukhanya.
Ngo-1914, uFranck noHertz, ababesebenza ngamandla e-ionization ama-athomu, basungula ucwaningo besebenzisa amazinga wamandla e-athomu ye-mercury. Ukuhlola kwakhe kwasebenzisa ama-electron nama-athomu e-mercury kuphela, ngaphandle kokusebenzisa noma yikuphi ukukhanya. Ngakho uBohr wathola ukuboniswa okungenakuphikiswa kwemodeli yakhe ye-athomu.
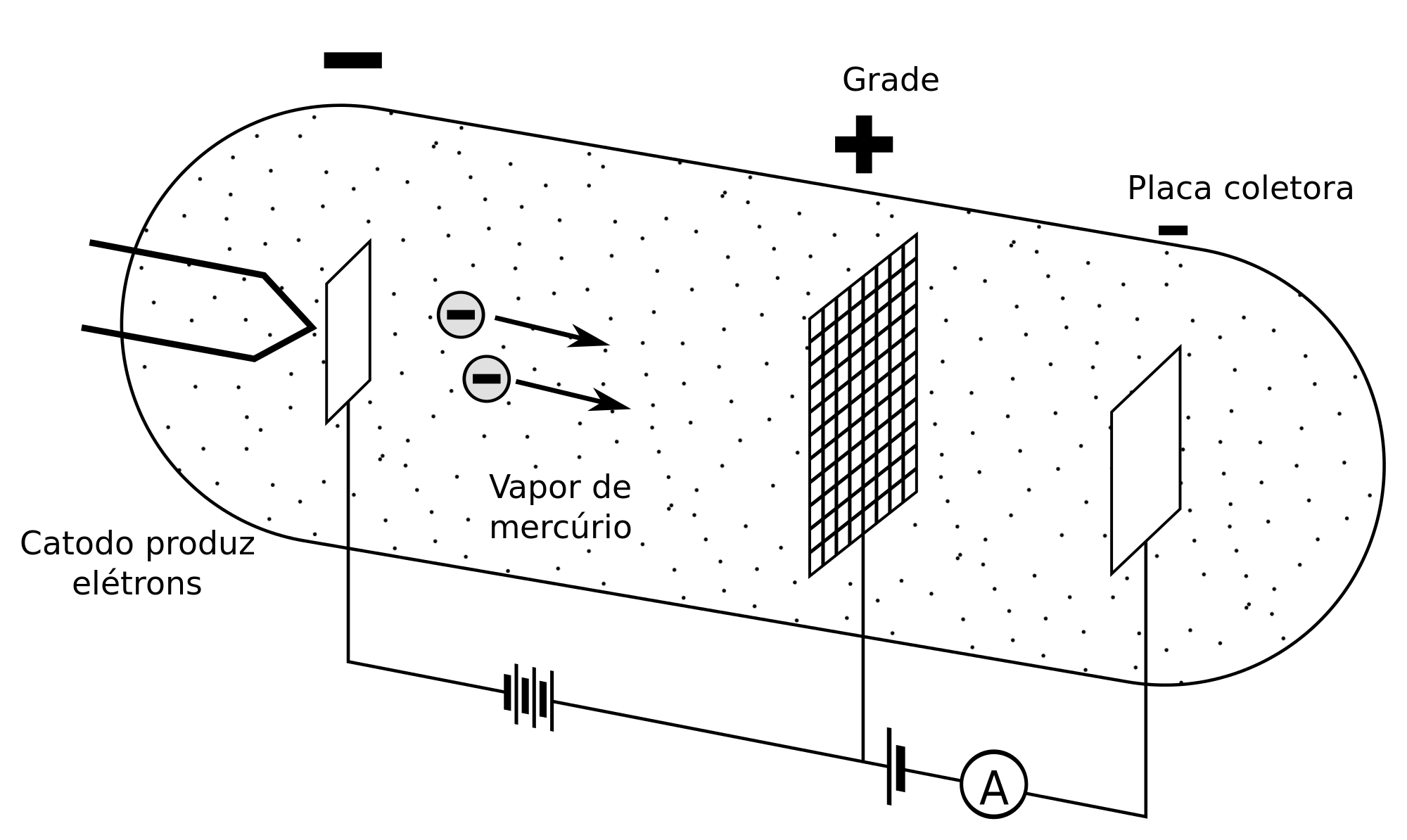
Ukuhlolwa kukaHertz ekusebenzeni
Ekuqaleni, ukuze babonise ukulinganisa kwamazinga wamandla, basebenzisa i-triode, eyenziwe nge-cathode, igridi ye-polarized kanye ne-anode, ekwazi ukudala i-electron beam ngaphakathi kwe-vacuum tube equkethe i-mercury esimweni segesi .
Base beqhubeka nokulinganisa ukuguqulwa kwamandla atholakala yi-anode ngokusho kwamandla e-kinetic aphethwe ama-electron, futhi ngaleyo ndlela bakwazi ukuthola ukulahlekelwa kwamandla ama-electron ngesikhathi lapho ukungqubuzana kwenzeka khona.
Material
Iqembu le-triode laliqukethwe ngaphakathi kwe-capsule yengilazi equkethe i-mercury. Kungenzeka ukwenza lokhu kuhlolwa emazingeni okushisa ahlukene futhi kubalulekile ukukwazi ukuqhathanisa le miphumela nesilinganiso ekamelweni lokushisa, lapho i-mercury izoba sesimweni se-liquid.
Uma i-mercury ishiselwa ezingeni lokushisa elingu-630 K, iba igesi. Kodwa ukuze ugweme ukufinyelela kulelo zinga lokushisa, kungenzeka ukusebenza ngokucindezela okuncishisiwe ngaphakathi kwe-capsule futhi kungashiswa kuze kufike ezingeni lokushisa eliphakathi kuka-100 no-200 °C.
Ukuze ama-electron akhishwe futhi ukuze ufinyelele isivinini esifanele, kufanele kusetshenziswe i-voltage ezoba phakathi kwe-cathode negridi, okuzoba i-acceleration voltage, ekhiqizayo. amaza omsakazo. Ngendlela efanayo, kungaba mnandi ukubeka i-voltage ngakolunye uhlangothi, phakathi kwe-anode negridi, ukuze unciphise ama-electron.
Imiphumela yokuhlolwa kwe-Hertz
Njengoba kuchaziwe ku I-biography kaHertz, umphumela walokhu kuhlola ukuthi kuzokwazi ukumela indlela umehluko ongaba khona ozobangelwa isiguquli se-voltage yamanje esibekwe ekuphumeni kwe-anode eguqukayo, ngokuhlobene nokukhipha okungaba umehluko wama-electron asuka ku-anode. i-cathode.
Ukuze uthole umehluko ongase ube khona ophansi, kuze kufike ku-4,9 V, okwamanje ogeleza ngeshubhu ukhula kancane kancane ngokwandisa umehluko ongaba khona. Ngamandla kagesi aphezulu insimu kagesi eshubhuni iyakhula futhi ama-electron azodonswa ngamandla engeziwe abheke kugridi yokusheshisa. Kulesi simo, ubona ukuthi ku-4,9 volts, okwamanje kwehla ngokuzumayo, cishe emuva ku-zero.
I-current izokhula kancane kancane uma i-voltage iqhubeka nokukhula, kuze kufike ku-9.8 volts, okuphindwe kabili ivolumu yokuqala yamanje esetshenzisiwe, futhi singabona ukuthi ukwehla okufanayo okungazelelwe kwenzeka ku-9.8 volts. Lolu chungechunge lwamaconsi amanje okwandayo okungaba ngu-4.9 volts luzobambelela ngokuphawulekayo kumandla okungenani angaba ngu-100 volts.
Ukuhunyushwa kwemiphumela yokuhlolwa kwe-Hertz
U-Franck no-Hertz bakwazi ukuchaza ukuhlola kwabo ngaphansi kwezimo zokungqubuzana okunwebekayo kanye nokushayisana kwe-inelastic kwama-electron. Ngamandla aphansi, ama-electron asheshayo athola inani elilinganiselwe lamandla e-kinetic. Lapho bebhekana nama-athomu e-mercury eshubhu lengilazi, benza ukungqubuzana okunwebekayo kuphela.
Lokhu kunesizathu sakho sokuba sesibikezelweni se-quantum mechanics esabonisa ukuthi i-athomu ayikwazi ukumunca noma yimaphi amandla kuze kube yilapho amandla okushayisana edlula inani elidingekayo ukuze kujabulise i-electron ebophezelekile ku-athomu eshiwo ungqimba lwamandla oluphakeme.
Ngokushayisana okunwebekayo kuphela, inani eliphelele lamandla e-kinetic ngaphakathi kwesistimu lihlala linjalo. Ngenxa yokuthi ama-electron anesisindo esingama-athomu amancane ngokuphindwe izikhathi eziyinkulungwane, lokhu kusho ukuthi ama-electron amaningi agcina amandla awo e-kinetic, eba amagagasi e-hertz. Amandla aphezulu aphumele ekushayeleni ama-electron engeziwe ukusuka kugridi kuya ku-anode futhi aphinde aphumelela ekwandiseni wamanje obonwayo, kuze kube yilapho amandla okusheshisa efinyelela ku-4.9 volts.
Amandla e-electronic excitation aphansi kakhulu i-athomu ye-mercury ingaba nezidingo ezingu-4,9 electron volts (eV). Esimeni lapho amandla asheshayo afinyelela ku-4.9 volts, i-electron ngayinye yamahhala imunca ncamashi u-4.9 eV wamandla e-kinetic, ngaphezu kwamandla ayo okuphumula kulelo zinga lokushisa, ngesikhathi lifika kugridi.
Ngenxa yalesi sizathu, ukungqubuzana phakathi kwe-athomu ye-mercury ne-electron yamahhala kungase kube i-inelastic ngaleso sikhathi, okungukuthi, amandla e-kinetic e-electron yamahhala angashintshwa abe amandla angaba namandla ngokujabulisa izinga lamandla le-electron ene-athomu ye-mercury. . Lapho wonke amandla ayo e-kinetic elahlekile, i-electron yamahhala ayikwazi ukunqoba amandla amancane angalungile ku-electrode yaphansi, futhi amandla kagesi ehla ngokushesha.
Lapho i-voltage inyuswa, ama-electron enza ukungqubuzana kwe-inelastic, alahlekelwe amandla e-kinetic angu-4.9 eV, kodwa abese ehlala esimweni esisheshayo. Ngale ndlela, okwamanje okulinganiswayo kukhuphuka futhi lapho amandla okusheshisa ekhuphuka, kusukela ku-4.9 V. Uma kufinyelelwa ku-9.8 V, isimo sishintsha futhi.
Ngaleso sikhathi, i-electron ngayinye inamandla adingekayo okuba yingxenye yokungqubuzana okubili kwe-inelastic, okwazi ukujabulisa ama-athomu amabili e-mercury, bese elahlekelwa wonke amandla e-kinetic. Yilokhu okuchaza ukwehla kwamanje okubhekiwe. Ezinkathini ezingu-4.9 volt, le nqubo izoziphinda, ngoba ama-electron azothola ukungqubuzana kwe-inelastic okwengeziwe.


