Have you heard of the photoelectric effect? Right here we offer you all the information that concerns the striking topic that emerges from quantum physics. Learn about its history, explanation, and concept, as well as some exponents who have made contributions to this branch of physics.
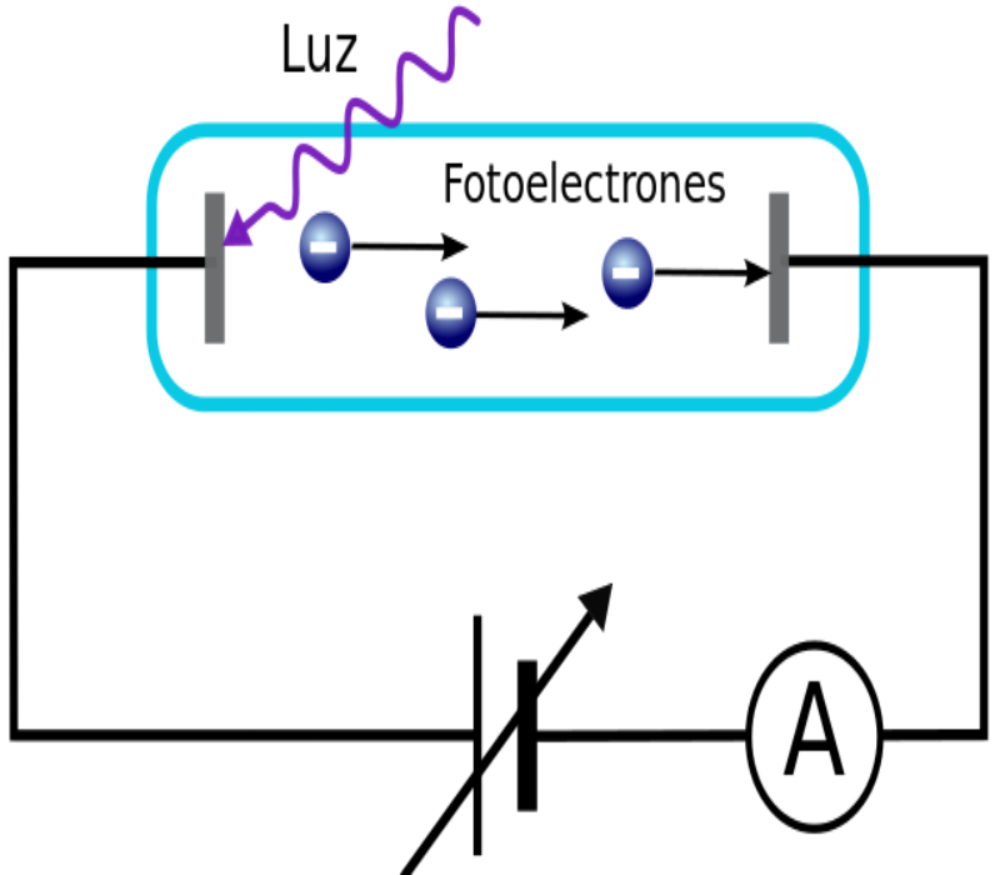
What is the photoelectric effect?
The photoelectric effect lies in the expression and manifestation of electrons, which is carried out through a conductor that can be an object that achieves the induction of electromagnetic radiation. This radiation is translated into perceptible light. Among some sheds of light we can find the following:
Photoconductivity
It plays a fundamental role thanks to the effects it carries out by increasing conductivity levels translated into electricity that light exerts. This experiment was exposed by the middle of the nineteenth century.
Photovoltaic effect
It is especially related to the fact that it triggers an effect that transforms light energy in contrast to electricity. Fact that is triggered in the year one thousand eight hundred and eighty-four.
Discovery
The discovery of the photoelectric effect is carried out thanks to the studies carried out by Heinrich Hertz in the year one thousand eight hundred and eighty-seven. Its observation is found under the approaches that involve a curve that bounces between 2 electrodes, and that are interconnected under a high voltage, that tends to reach greater distances when illuminated by UV light, which is completely different than when it is at dark.
The first proof of this theoretical point was outlined through the definition or description proposed by Albert Einstein on the photoelectric effect, reaching the conclusion that the particle that corresponds to light is called a photon. The basis for the creation of this light-based theory was used by Einstein thanks to the eminent studies of Planck. Who made some efforts to show the existence of how many.
La Biography of Max Planck shows us the incursion of this scientist in the world of physics, in addition to evidencing certain recognitions that were granted thanks to the studies carried out on the quanta of action. Taking into account that this theory opened the doors to the path of quantum physics in a fast and fluid way.
El photoelectric effect It is in contrast to X-rays. Taking into account that photons achieve the transfer of electrons in this process of electromagnetic radiation, while in the case of X-rays it was not until several studies that the composition on which X-rays are generated. That by the year 1985 the effects and use of said radiation called rays, by the scientist Wilhelm Rotge, is discovered.
photons
The photons they are represented by energies that are delimited by a type of light frequency in the form of a wave. If we find ourselves with the case of an atom, which finds itself absorbing a certain amount of energy that emerges from a certain photon, it has large energetic amounts that allow it to throw an electron from the material in question, to later go towards a specific path that ends in a certain space.
Having happened the above, the electron is repelled from the material. In the opposite case. If the energy that the photon emanates does not have enough strength, the electron does not have the agility to escape or escape from the material in question.
For its part, it does not depend on the changes generated by the force of light that the energy present in the photon be modified, only the number of electrons that manage to escape out of the space on which they are found have the power to do so. , thanks to the force that the electrons emit, it is clear that it is not dependent on the radiation it reaches, but on the frequency emitted.
In general, not all electrons are able to be expelled by the electron. photoelectric effect, It is taken into account that the first to come out are those that do not usually need extreme force to achieve successful expulsion. In a dielectric insulator, some electrons with large amounts of energy can be seen in the valence band.
In the case of metal, we usually find the electrons in front of a wide band that gives a great conduction.
Therefore, through the semiconductor it is possible to show the electrons that transmit a large amount of energy. In terms of conductors of this type, few electrons are usually found in the band that generates conduction.
When we talk about room temperature we usually find some electrons with large amounts of energy, which have been found very close to the Fermi levels. There is an energy that an electron must contain in order to reach a Fermi level, this is known as working fusion, while the minimum frequency needed for radiation to expel an electron is called the threshold frequency.
The assessment of said energetic quantity is versatile, and never constant, this of course, depending at all times on the material and its atomic layers. Some metallic materials such as calcium and cesium have very low work performance. For this reason, it must be absolutely strict that the material is clean as far as the atoms are concerned.
Explanation
The photons that have light rays, in turn have a peculiar energy, which is established by the frequency that the light provides. Through the photoemission procedure, if it is the case that an electron manages to absorb the energy of a photon and the photon has the energy even greater than the work function, the electron will be expelled from the matter.
When the energy of the beam increases, there is no change in the energies of the photons, there is only a change in the numerical quantity of the photons. Therefore, the obvious conclusion is that the energy of each electron will never depend on the intensity or strength that the light provides, but on the energy that each photon produces.
Strictly all the energy that the photon acquires has to be attracted and in turn must be used to achieve the release of an electron that is bound to an atom. In this case, that said energy containing the photons that manages to consume one of these parts, unties the electron from the atom and the rest is converted as a contribution of the kinetic energy as part of the electron, ending up in a free particle.
Albert, for his part, did not have as a goal the specific study of the causality generated by the electrons in the radiation of some metals, which later became kinetic energy, however he made his pertinent observations.
He found the explanation of the behavior exerted by radiation. Through this action he set out to explain by observation the number of electrons that left the material, taking into account that the frequency played a fundamental role in the actions carried out.
History
In the world of physics we managed to delimit the history of some discoveries that are recorded on exact dates, thanks to the study of some Important scientists who contributed with various studies and theories that today have helped to explain some phenomena of physics, among the scientists that we can mention we find:
Heinrich Hertz
This scientist managed to carry out the first study on the observation of the photoelectric effect in the year one thousand eight hundred and eighty-seven. The instruments under which he carried out this experiment are based on a coil on which a spark could be made as a guarantee that it would function as a receiver of electromagnetic waves.
In order to obtain a complete vision of the panorama, and in turn to achieve the observation of the spark, he enclosed the receiver in a black box or container. Given this, an absorption of UV light was carried out, which easily provided the jump of electrons. And in turn, the force that contained the spark endowed with electricity that the receiver produced was directly evidenced. The scientist published said experiment even without explaining the phenomenon.
Joseph John Thomson
By XNUMX, the scientist Thomson was preparing the foundations for a study specifically on cathode rays. Under the influence of Maxwell, the scholar concludes that the cathode rays were rooted in a flow of particles that were found with various negative charges, to which he gives the name of corpuscles, and that finally they are given the name of electrons.
Joseph took the basis of his experiment on a totally closed metal plate in a vacuum tube, exposing said element to light with a complete difference in terms of wavelength. The scientist believed that the electromagnetic field gives some resonances with the electric field, and that a corpuscle endowed with an electric charge is emitted through it.
The intensity that was present in said current endowed with electricity was very variable in the face of the intense levels that the light produced. This meant that as the light increased, the current also increased. Its translation is carried out thanks to the fact that the radiation that has a higher frequency, in turn also produces particles with greater kinetic energy.
Philipp Lenard
For the year nineteen hundred and two, this scientist carried out a study on the photoelectric effect in which he manifested the energetic variation of the electrons, concluding that they play a fundamental role with the frequency of the incident light.
Albert Einstein
In nineteen hundred and five, the scientific formulation of the famous theory of relativity is carried out, which the scientist proposes under prescriptions that were based on mathematical and numerical bases, which allowed the understanding of some procedures. The emission of electrons was linked to the production and absorption of light quanta, which were later called photons.
In 1905, the same year that he held a class on the theory of relativity, Albert Einstein proposed an investigation in which he exposed a phenomenon that seemed to work correctly, in which the emission of electrons was produced by the absorption quanta of light, a fact that would later be called photons.
In an article titled A Eucharistic Viewpoint on the Production and Transformation of Light, he showed how the idea that discrete particles of light could generate the photoelectric effect and also showed the presence of a characteristic frequency for each material below which had no effect. For this explanation of the photoelectric effect Einstein would receive the Nobel Prize in Physics in 1921.
Taking Einstein's theory into account, the energy with which the electrons fled the cathode at the same time as they rose steadily, through the frequency of the incident light, away from the intense form of energy. Greatly, such an effect had not been seen in ancient times. The experimental demonstration of this aspect was carried out in 1915 by the American physicist Robert Andrews Millikan.
Finally, each and every one of the scientists mentioned above have made great contributions to the study and discovery of the photoelectric effect. Thanks to which today the knowledge, and the theoretical approaches have been very well received.
Today this incredible photoelectric effect counts as a mechanism that can be found in various electronic equipment. His discovery was really important thanks to the studies that were carried out in order to know some effects that light has.
Being the studies of said scientists, contributions that managed to make a great difference in the world of physics. Thanks to this, quantum physics is a scientific branch that obtained a great level of prestige, which progressively developed with great impetus and interest.
wave-particle duality
This phenomenon is the physical effect that was discovered in the first instance together with other spectra of the same characteristics. He originated the discovery of the so-called wave-particle that is a component of quantum mechanics. Light behaves like waves, being able to produce interference and diffraction as in Thomas Young's double slit experiment, but it exchanges energy in a discrete way in energy packets, photons, whose energy depends on the frequency of electromagnetic radiation.
These ideals managed to build a theory of electromagnetic radiation with extremely clear and defined bases, since through it, explanations arose about other terms that are involved in the functions that radiation carries out.
Photoelectric effect today
Today the photoelectric effect is usually the complete basis that can be found before the energetic levels that are manifested in a photovoltaic way, this type of effect is usually found in thermoelectric industries, as it is manifested in some sensitive systems that contain cameras digitized.
In other elements, the photoelectric effect is present in everyday household appliances, most of which are made up of a very potential material, such as copper, these elements achieve the production of potential electrical currents.
We can also find this phenomenon in bodies that are exposed to the reflections of the Sun for a considerable period of time. The dust particles that make up the surface of the Moon, upon receiving this light directly, are charged with positive energy, this is thanks to the impact of photons. These tiny fragments, being charged, repel each other, thus rising and forming a tenuous atmosphere.
Natural satellites also receive a positive electrical charge and fill the surface that is illuminated by the Sun, however, in the darkest region, it is charged with negative energy. It should be noted that it is necessary to take this eventuality of energy accumulation into account.
Finally, the discovery of the photoelectric effect brought with it the improvement that over time helped us to understand in a magnificent way the deep structure that the world presents. In turn, the advances that triggered its effect, translate into the following technological advances:
- Transmission of animated images
- cinema progress
- TV
- Heavy machinery, used in industrialization processes.
In the area of electricity, the photoelectric effect achieves incredible results, since public lighting is possible thanks to its use. Taking into account that many of the machines that perform this task do not need to be monitored or supervised by any worker or operator, since this effect automatically turns on and off the lights that illuminate the avenues or streets of any place.
Without a doubt, this effect is really complex to understand, however, its studies were quite in-depth in ancient times, thanks to scientists who made quite interesting and concrete contributions, which have been fully recognized at the scientific level.