Do you know what the hertz experiment? It was a study carried out for the first time in 1914 by the scientists James Franck and Gustav Ludwig Hertz, whose purpose was to establish the quantization of the energy levels of the electrons present in atoms.
Franck and Hertz experiment
Hertz's experiment was able to confirm Bohr's quantum model of the atom, proving that atoms were only capable of absorbing specific amounts of energy called quanta. For that reason, this is one of the essential experiments for quantum physics. For this research, Franck and Hertz were awarded the Nobel Prize in Physics in 1925.
History, Who was Hertz?
In the year 1913, Niels Bohr advocated the existence of a new model of the atom, later called the Bohr Atomic Model, and proposed the existence of electron orbits, which had as a model the Rutherford Atomic Model, much like a planetary system. Using his model, he proposed four postulates, one of which was related to the quantization of electron orbits.
In this way, the first experiments aimed to be able to verify this quantization. In the first experiments, light was used, since at that time it was known that light was made up of quanta of energy. For this reason, Bohr is criticized for the fact that the results of the quantization of the orbits, and therefore, of the quantization of the energy states of the electrons of the atom, had their origin only in the quantization of light.
In 1914, Franck and Hertz, who were working on the ionization energies of atoms, devised an experiment using the energy levels of the mercury atom. His test only used electrons and mercury atoms, without using any light. Bohr thus obtained the irrefutable proof of his atomic model.
Hertz's experiment in practice
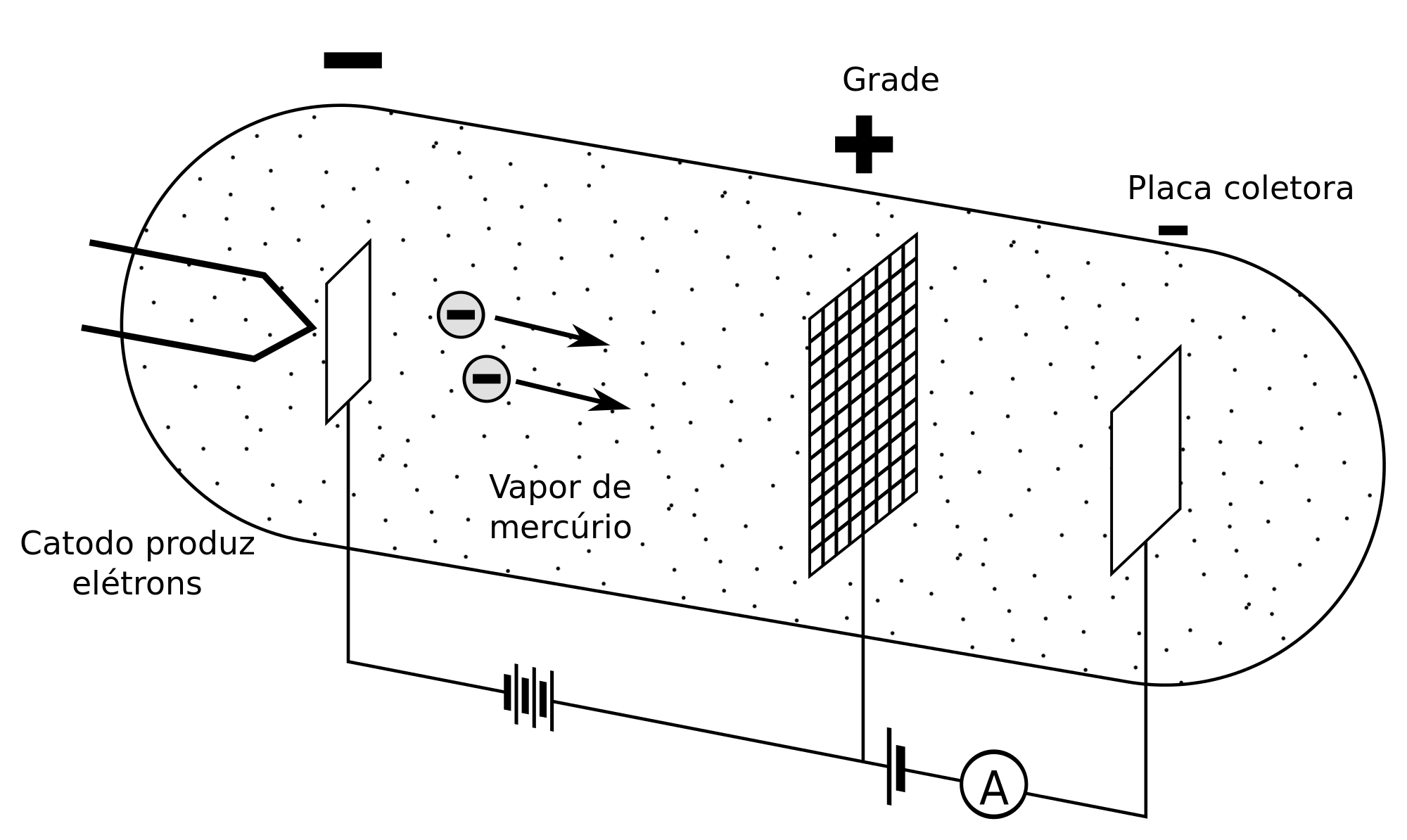
At first, in order to demonstrate the quantization of energy levels, they used a triode, made up of a cathode, a polarized grid and an anode, which is capable of creating an electron beam inside a vacuum tube. containing mercury in a gaseous state.
They then proceeded to measure the modification of the current received by the anode according to the kinetic energy possessed by the electrons, and thus they were able to deduce the loss of energy of the electrons at the moment in which the collisions occurred.
Material
The triode group was contained within a glass capsule containing mercury. It is possible to carry out this experiment at different temperatures and it is important to be able to compare these results with a measurement at room temperature, in which the mercury will be in a liquid state.
When mercury is heated to a temperature of 630 K, it becomes a gas. But to avoid having to reach that temperature, it is possible to work with a reduced pressure inside the capsule and it can be heated to a temperature that ranges between 100 and 200 °C.
For the electrons to be extracted and for you to reach a relevant speed, a voltage must be used that will be located between the cathode and the grid, which will be an acceleration voltage, producing radio waves. In the same way, it may be interesting to place a voltage in the opposite direction, between the anode and the grid, in order to slow down the electrons.
The results of the Hertz experiment
As explained in Hertz's biography, the result of this experiment is that it will be possible to represent the way in which the potential difference that will result from a current-voltage converter that is placed at the anode output evolves, in relation to the extraction potential difference of electrons from the cathode.
To obtain low potential differences, down to 4,9 V, the current flowing through the tube increases steadily with increasing potential difference. With the higher voltage the electric field in the tube increases and the electrons will be drawn with more force towards the acceleration grid. In this case, it is observed that at 4,9 volts, the current drops suddenly, almost back to zero.
The current will increase steadily if the voltage continues to increase, until 9.8 volts is reached, which is exactly twice the first volume of current used, and we can see that a similar sudden drop occurs at 9.8 volts. This series of current drops for increments of about 4.9 volts will hold observably down to potentials of at least about 100 volts.
Interpretation of the results of the Hertz experiment
Franck and Hertz were able to explain their experiments under conditions of elastic collision and inelastic collision of electrons. At low potentials, the accelerated electrons acquired only a moderate amount of kinetic energy. When they confronted the mercury atoms in the glass tube, they made only elastic collisions.
This has its reason for being in the prediction of quantum mechanics that indicated that an atom is not capable of absorbing any energy until the energy of the collision exceeds the value required to excite an electron that is bound to said atom at a higher energy layer.
For only elastic collisions, the absolute amount of kinetic energy within the system remains the same. Because electrons have a mass that is about a thousand times lighter than less massive atoms, this means that most of the electrons retained their kinetic energy, becoming hertz waves. Higher potentials resulted in driving more electrons from the grid to the anode and also succeeded in increasing the observed current, until the acceleration potential reached 4.9 volts.
The lowest electronic excitation energy a mercury atom can have needs 4,9 electron volts (eV). In the case where the accelerating power reached 4.9 volts, each free electron absorbed exactly 4.9 eV of kinetic energy, above its rest energy at that temperature, by the time it reached the grid.
For this reason, a collision between a mercury atom and a free electron can be inelastic at that time, that is, the kinetic energy of a free electron can be turned into potential energy by exciting the energy level of an electron that has a mercury atom. When all its kinetic energy is lost, the free electron is unable to overcome the slight negative power at the ground electrode, and the electric current drops precipitously.
When the voltage is increased, the electrons form an inelastic collision, lose their kinetic potential of 4.9 eV, but then remain in an accelerated state. In this way, the current that is measured rises again when the acceleration potential is increased, starting from 4.9 V. When 9.8 V is reached, the situation changes again.
At that moment, each electron has the necessary energy to be part of two inelastic collisions, which manages to excite two mercury atoms, and then lose all their kinetic energy. This is what explains the observed current decreases. In the 4.9 volt intervals, this procedure will repeat itself, because the electrons are going to experience a further inelastic collision.


